Abstract
Introduction: Vodobatinib is a novel third generation TKI effective against wild-type and mutated BCR-ABL1 (except T315I) with limited off-target activity. We present updated results from the Phase 1 dose-escalation (DEs) and expansion (DEx) study in CML and Ph+ALL patients (pts) failing ≥ 3 prior TKIs (< 3 prior TKIs if approved TKI is not clinically advised or available); patients with T315I are not eligible (NCT02629692).
Methods: This is an open-label, phase 1, multicentre, 3+3 study evaluating maximum tolerated dose (MTD), safety and efficacy of vodobatinib administered once daily in 28 day cycles (dose range: 12 to 240 mg). MTD was established at 204 mg. DEx study enrolled chronic phase CML (CP-CML) patients at 174 mg dose of vodobatinib. Treatment continued until unacceptable toxicity, disease progression, consent withdrawal or death. Adverse events were assessed using NCI-CTCAE v4.03.
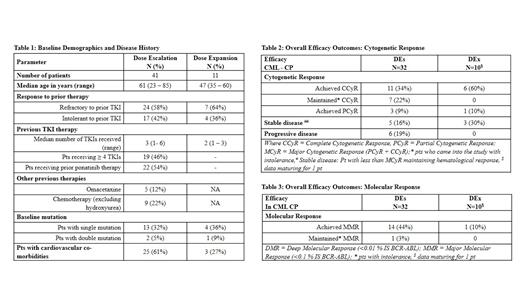
Results: As of 15 Jul 2021, 52 pts are enrolled in DEs and DEx cohorts. Forty one of these pts received doses from 12 to 240 mg in the DEs cohort;32 chronic phase (CP-CML), 3 accelerated phase CML (AP-CML), 4 blast phase CML (BP-CML), 2 Ph+ ALL. Eleven CP-CML pts were enrolled in DEx cohort at 174 mg dose. The baseline demographics and disease history are represented in Table 1.
Efficacy: Of the 32 CP-CML pts enrolled in DEs: At baseline, 21 (65%) pts had no cytogenetic response, 4 (12.5%) were in PCyR, 7 (22%) were in CCyR. On vodobatinib therapy, 11(34%) pts achieved CCyR, 3 (9%) achieved PCyR and 7 (22%) maintained baseline CCyR. Baseline major molecular response (MMR) was present in 1 (3%) pt; and 14 pts (44%) achieved MMR on study. Of the remaining 11 pts, 5 (16%) had haematologically stable disease (no CyR and molecular response) and 6 (19%) had disease progression (cytogenetic or hematological) as their best response (Table 2 and 3). Seventeen CP-CML pts had prior ponatinib treatment, of which 11 (65%) had MCyR (4 achieved CCyR, 4 maintained CCyR, 3 achieved PCyR); while 8 (47%) achieved MMR. In the remaining 15 pts ponatinib naïve CP-CML: 10 (66%) had CCyR (7 achieved CCyR, 3 maintained CCyR); with 7 (47%) with MMR (6 achieved, 1 maintained).
Two of the 3, AP-CML pts had baseline hematological response (CHR) with absence of cytogenetic and molecular response. The 3 pts further deepened their responses with 1 pt achieving CCyR with MMR and 2 pts in PCyR. Of the 4 BP-CML pts, 2 achieved CHR and 2 patients had disease progression as their best response; Of the 2 Ph+ ALL pts, 1 pt maintained CCyR and MMR while the other reported disease progression as the best response. Median duration of treatment overall was 23 (0.5-51) months [CP-CML 23 (0.5-51); AP-CML 36 (9-40); BP-CML 3 (0.5-18) and Ph+ ALL 4 (0.7-7.3) months]. Twenty one pts continue in study.
In the DEx cohort, 1 of the 11 CP-CML pts was in PCyR at baseline. No pts had molecular response. Of the 11 patients, 6 (54 %) pts achieved CCyR, 1(10%) pt achieved PCyR. MMR was achieved by 1 pt (10%). Data is maturing for 1 pt. Median duration of treatment is 16 (0.3-19) months and 10 pts continue in study.
Safety: Forty nine of 52 pts reported at least 1 TEAE. Most common any grade TEAEs included thrombocytopenia (33%), cough (19%), anaemia & diarrhoea (17% each).
Thirty one pts (60%) reported Grade 3 and 4 treatment emergent AEs: most common were thrombocytopenia (15%), neutropenia and anaemia (12%), increased amylase and lipase (8% each). Ten (19%) pts reported cardiovascular TEAEs (Grade 1: angina pectoris, palpitations, ventricular extra-systoles, arteriosclerosis, hot flush, hypotension, intermittent claudication; Grade 2: hypertension, hypotension; Grade 3: cardiac failure congestive, hypertension); with a Grade 2 hypertension being vodobatinib related. Nineteen pts (37%) reported SAEs; vodobatinib related SAEs were reported in 3 pts (fatal intracranial haemorrhage (ICH), Grade 2 back pain and Grade 3 amnesia reported in 1 pt each). There were 5 deaths on study: 1 was related to use of vodobatinib (1 ICH, confounded by disease progression to blast phase that included extra- medullary sites) and the remaining unrelated (1 sudden death, 1 disease progression, 1 pneumonia fungal, 1 suspected COVID-19).
Conclusion: Vodobatinib continues to be associated with favourable long term safety and efficacy in heavily pre-treated CML failing ≥ 3 prior TKIs, including ponatinib. Phase 2 study evaluating vodobatinib in pts failing at least 3 prior lines of therapy, including ponatinib, is ongoing.
Cortes: Novartis: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sun Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Kim: Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding; ILYANG: Research Funding; Takeda: Research Funding. Alvarado: MEI Pharma: Research Funding; Daiichi-Sankyo: Research Funding; Sun Pharma: Consultancy, Research Funding; CytomX Therapeutics: Consultancy; Jazz Pharmaceuticals: Research Funding; BerGenBio: Research Funding; Astex Pharmaceuticals: Research Funding; FibroGen: Research Funding. Nicolini: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations, expenses, Research Funding; Incyte Biosciences: Honoraria, Other: travel, accommodations, expenses, Research Funding, Speakers Bureau; BMS: Honoraria; Sun Pharma Ltd.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Apperley: Incyte, Pfizer: Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb, Novartis: Honoraria, Speakers Bureau. Deininger: Fusion Pharma, Medscape, DisperSol: Consultancy; Novartis: Consultancy, Research Funding; SPARC, DisperSol, Leukemia & Lymphoma Society: Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding. de Lavallade: Bristol Myers Squibb: Research Funding; Incyte: Honoraria, Research Funding; Novartis: Speakers Bureau. Charbonnier: Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Gambacorti-Passerini: Pfizer: Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy. Mauro: Novartis: Consultancy, Research Funding; Takeda: Consultancy; Pfizer: Consultancy; Sun Pharma / SPARC: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Vandenberghe: Pfizer: Research Funding; Bristol Myers Squibb/Celgene: Consultancy; Miltenyi Biotec: Consultancy; Novartis: Consultancy; Janssen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy; Gilead Sciences: Consultancy, Other: Travel support. Radhakrishnan: Intas Pharmaceuticals: Research Funding; Emcure Pharmaceuticals: Research Funding; Dr Reddy's Laboratories: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen India: Honoraria; Cipla Pharmaceuticals India: Research Funding; Bristol-Myers-Squibb India: Membership on an entity's Board of Directors or advisory committees, Research Funding; Aurigene: Speakers Bureau; AstraZeneca India: Honoraria, Speakers Bureau; NATCO Pharmaceuticals: Research Funding; Novartis India: Membership on an entity's Board of Directors or advisory committees; Roche India: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Yao: Sun Pharma: Current Employment. Inamdar: Sun Pharma Advanced Research Company: Current Employment. Sreenivasan: Sun Pharma Advanced Research Company: Current Employment. Dillu: Sun Pharma Advanced Research Company: Current Employment. Chimote: Sun Pharma Advanced Research Company: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal